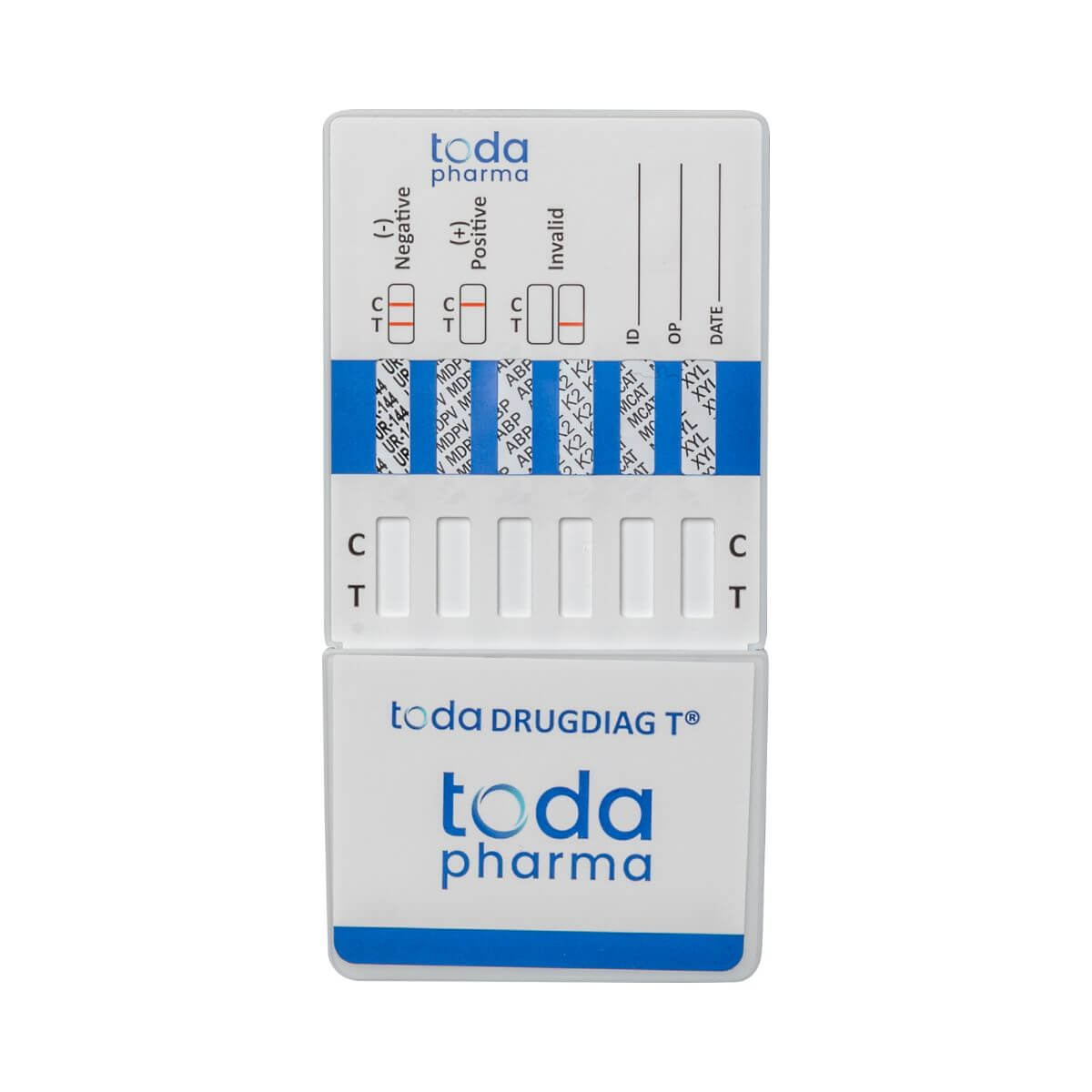
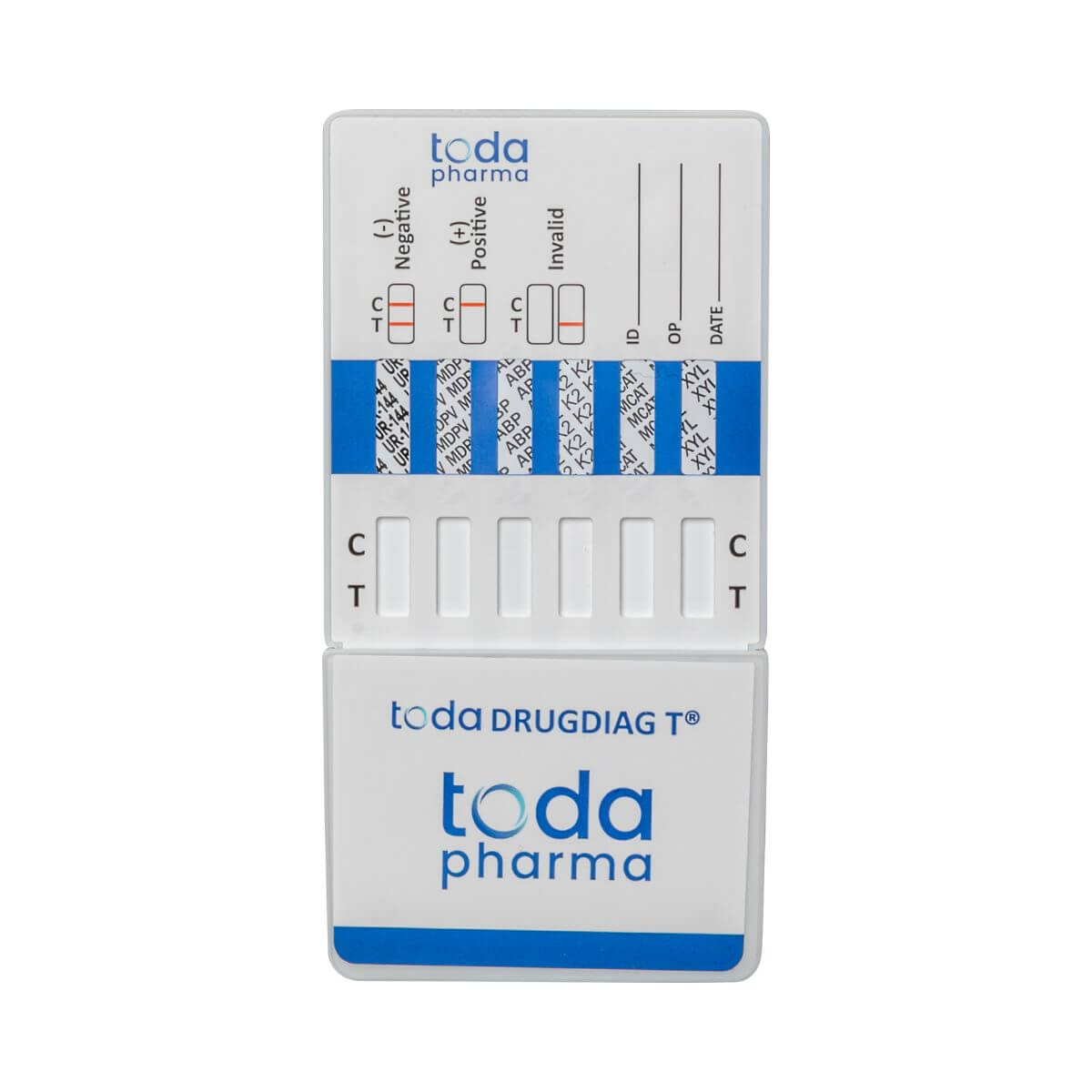
Drugdiag® 6T Urine Test - New Synthetic Drugs
Laboratoire Toda Pharma

Expédition Offerte
dès 49€

Fabriqué en France
Norme CE

Qualité Médicale
ISO 13485
Couldn't load pickup availability

Advanced protection against over 100 hazardous substances
The Drugdiag® 6T urine test developed by Toda Pharma allows for the rapid detection of new synthetic drugs most commonly encountered in party or high-risk environments. Thanks to its recognized medical precision , it identifies substances that are often undetectable by conventional tests .
🔬 Substances reliably detected:
- UR-144 / K4 (synthetic cannabinoids)
- MDPV (synthetic cathinone)
- ABP (AB-PINACA / AB-FUBINACA)
- K2 (mixture of synthetic cannabinoids)
- MCAT (mephedrone)
- Xylazine (diverted veterinary sedative)
💡 Drugs detected: more than 100 known products
Among them:
Spice, Black Mamba, Buddha Blue, PTC, 3-MMC, 4-MMC, Meow Meow , and other psychoactive substances associated with chemical submission , party doping , or new addictions .
✅ The advantages:
- Results in 5 minutes – immediate screening
- Professional reliability – used in prevention, in business and in festive environments
- Non-invasive – simple urine test
- Current standards – CE compliant, laboratory tested
- Extended detection – substances not covered by conventional tests
This test is an essential tool for professionals , associations, establishments open to the public, and all structures wishing to act against emerging synthetic drugs , often involved in cases of chemical submission or uncontrolled consumption .

Label "Used by the French Army "
Recognized for its reliability, the Toda Pharma laboratory carries the prestigious label "Used by the French Army" . A guarantee of trust and quality which also makes it the preferred choice of many health professionals and individuals .

✅ The drug screening test most used by healthcare professionals
Shelf life: With a validity of 24 months , our tests guarantee precise and reliable results over the long term, meeting the highest requirements.
Important : This product is designed for professional and personal use , in compliance with current regulations.

Our Clients
MOST FREQUENTLY ASKED QUESTIONS
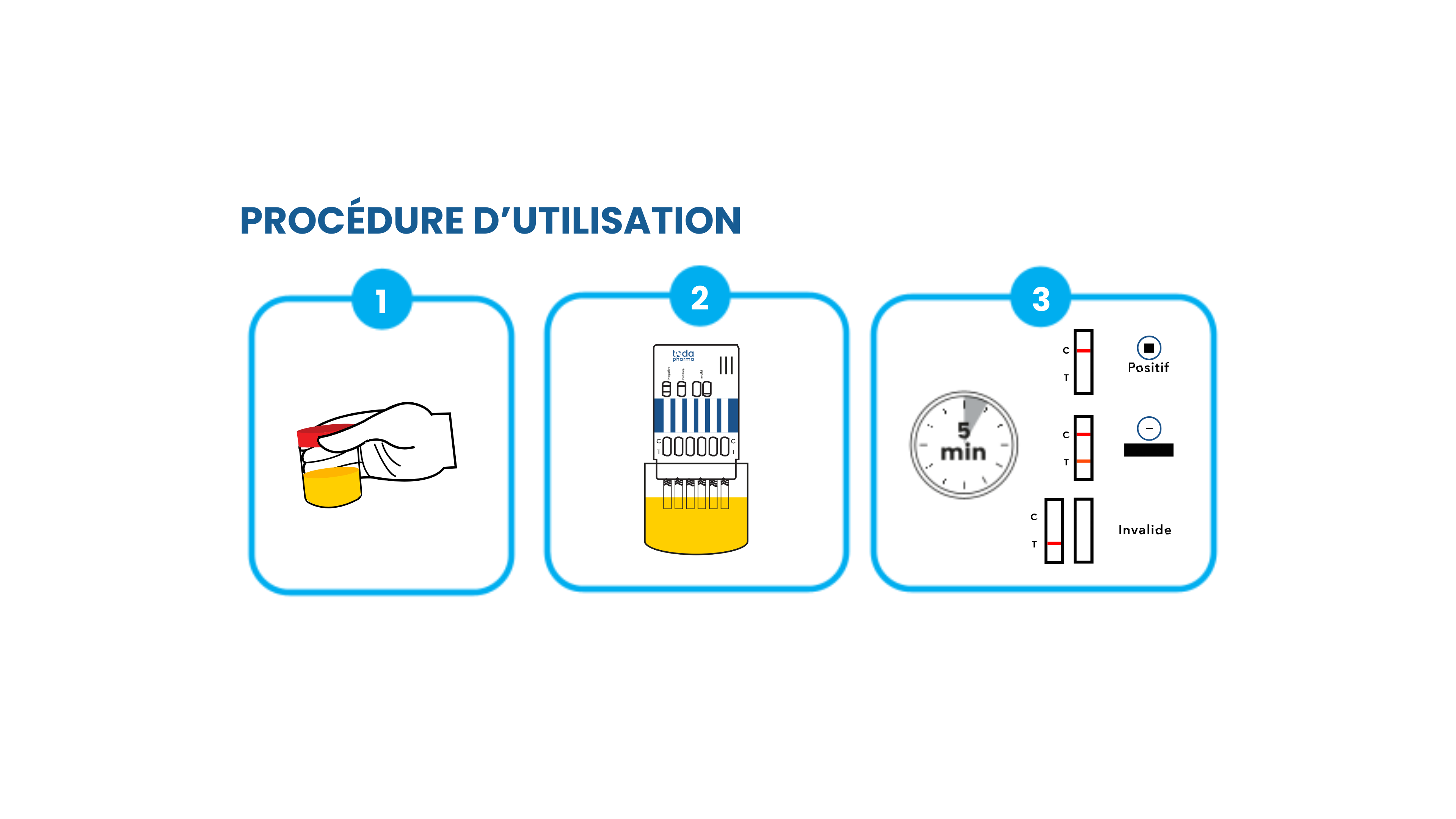
How do Drugdiag® T urine tests work?
TODA Pharma Drugdiag T® urine tests are designed for simple, one-step use . Simply dip the test directly into the urine and wait approximately 3 minutes to interpret the results. The reading areas and instructions are clearly marked on the test, making it quick, intuitive, and accessible to everyone .
How reliable are the Drugdiag® T tests for new synthetic drugs?
Drugdiag® T New Synthetic Drugs tests are manufactured according to the strictest standards
to guarantee their 96% reliability and accuracy. They are used by
healthcare professionals and law enforcement to carry out
Drug screenings. You can trust the reliability of our tests to obtain accurate results.
What substances can be detected by Drugdiag® T urine tests? New synthetic drugs?
TODA Pharma Drugdiag T® New Synthetic Drugs offers a range of dip-in urine tests , enabling rapid and reliable detection of multiple substances, including:
- THC (cannabis) — 50 ng/ml
- K2 (Spice, synthetic cannabinoids) — 50 ng/ml
- ABP (e.g. AB-PINACA) — 10 ng/ml
-
UR-144 (Buddha Blue, PTC) — 25 ng/ml
- MCAT (mephedrone, 3-MMC etc) — 500 ng/ml
- MDPV (synthetic cathinone) — 1,000 ng/ml
- XYL (xylazine) — 1000 ng/ml
- MDMA (ecstasy) — 500 ng/ml
- COC (cocaine) — 300 ng/ml
- AMP (amphetamine) — 1,000 ng/ml
- MET (methamphetamine) — 1000 ng/ml
- MOP (opiates) — 300 ng/ml
These tests are designed to be simple, quick and accurate , offering an effective solution for professionals, businesses, healthcare facilities and individuals concerned with prevention and responsible screening.
Do these tests comply with safety and quality standards?
Yes, TODA Pharma Drugdiag® urine tests are CE and ISO 13485 certified , ensuring compliance with the highest quality and safety standards.
What is the shelf life of Drugdiag® T urine tests for new synthetic drugs?
Drugdiag® T urine tests for novel synthetic drugs have a shelf life of up to 24 months, usually indicated on the product packaging. It is recommended to store the tests under appropriate conditions, away from direct sunlight and at a specified temperature.
Where are Drugdiag® T tests manufactured for new synthetic drugs?
We are proud to offer drug screening tests of the highest quality , also manufactured in France in Strasbourg (67) .
By working with local partners like Toda Pharma , we ensure products meet the highest standards of safety and efficacy.
Our devices are all CE marked and comply with ISO 13485 standards.
for quality management, are subject to monitoring
Rigorous quality controls. This guarantees performance.
optimal and unparalleled accuracy of results.
Are Drugdiag T tests for new synthetic drugs suitable for businesses and institutions?
Yes, we offer solutions adapted to the needs of businesses, schools, and other institutions.
Contact our team to obtain a personalized quote on our products as well as our services concerning awareness and prevention in professional or festive environments.
How can I interpret the test results?
TODA Pharma Drugdiag® urine test results are interpreted based on the lines or symbols clearly marked on the test. Detailed instructions are provided in the user manual included with each kit.
Can these urine tests be used to detect unintentional substance use?
Yes, Drugdiag® T urine tests for new synthetic drugs play an essential role in preventing the involuntary consumption of unwanted substances, offering rapid and accurate detection.
Please note that this information is provided for informational purposes only. If you have any doubts or concerns, it is always recommended that you consult a qualified healthcare professional.

French manufacturing and medical quality
Toda Pharma, a French manufacturer of drug screening tests, was one of the first European laboratories to develop a rapid serological test for the detection of coronavirus in February 2020 and was validated by the National Reference Center (CNR), the Pasteur Institute and the French Ministry of Solidarity and Health .
A study was carried out in 3 hospitals by different operators on 3 separate batches of each drug at concentrations of 0ng/ml, +/- 25% and +/- 50% of the detection threshold of the drug in question. All results were in line with expectations. The numerical tables of all the results are available to users upon request.

UAF Label – Reliability recognized by experts
Used by the French army for its screening checks, guaranteeing maximum precision and reliability.
The UAF label certifies that this test meets the strictest standards, validated by the French armed forces. It offers a guarantee of quality and performance , ensuring screening as reliable as the tests used in military institutions.

The CE Standard
CE certification guarantees that our products meet the essential safety, health and environmental protection requirements set by the European Union. It certifies that our breathalyzers and drug screening tests comply with European standards, thus offering safe and reliable use. By choosing a CE certified product, you can be sure that it has been rigorously tested and meets the quality and safety standards in force on the European market.

ISO 13485 Standard
ISO 13485 is an international standard that certifies the quality of medical devices. This certification attests that our manufacturing processes meet the highest standards of quality and risk management. Our screening tests are designed and produced under strict conditions to ensure their reliability, safety and effectiveness. By opting for our ISO 13485 certified products, you can be sure that you are using equipment that complies with the requirements of the medical sector, for precise and consistent results.

Holder of the Cœur Alsace label
Toda Pharma is honored to receive the "Cœur Alsace" label, a symbol of excellence and attachment to our region. This prestigious label recognizes our commitment to quality, innovative local production that complies with the strictest standards. Based in Strasbourg, Toda Pharma embodies Alsatian know-how in the service of public health and safety.